Progressive multifocal leukoencephalopathy (PML) is a neurological disease caused by the JC virus. PML is a demyelinating disease that primarily affects the central nervous system.
Progressive multifocal leukoencephalopathy (PML) is a neurological disease caused by the JC virus. PML is a demyelinating disease that primarily affects the central nervous system. Although it remains a latent infection for most people, it is particularly virulent in immunosuppressed patients such as AIDS, recipients after solid organ and bone marrow transplantation, malignancies, and chronic inflammatory conditions. It is also known to cause disease in patients receiving antiretroviral therapy.
This is because the treatment can cause immune reconstitution.These cases of PML are called PML-IRIS. Certain monoclonal antibodies, particularly natalizumab, which is used to treat multiple sclerosis, can also cause PML. This is an important difference to consider when assessing neurological symptoms in patients with these particular risk factors.
Etiology
JC virus is a DNA virus of the Polyomaviridae family. The virus remains dormant in most immunocompetent hosts and rarely manifests pathologically. However, in immunocompromised hosts, the combination of reduced host cell responses and viral reactivation as a result of genetic recombination results in active disease.
Progressive multifocal leukoencephalopathy can occur as part of immune reconstitution syndrome. The diagnosis of PML IRIS should be considered in AIDS patients receiving antiretroviral therapy who present with altered mental status associated with focal neuropathy or non-enhancing lesions. This condition can occur between 1 week and 26 months after starting HAART.
PML is known to occur after treatment with monoclonal antibodies, particularly natalizumab, rituximab, efalizumab, and eculizumab. These drugs are used to treat autoimmune diseases such as multiple sclerosis, Crohn's disease, psoriasis, and lupus.
Clinicians should be aware of the emergence of neurological dysfunction in patients taking these drugs. In particular, natalizumab is recommended as monotherapy for his MS. Moreover, increased frequency of natalizumab infusions increases the likelihood of developing PML.
Epidemiology
Most cases of progressive multifocal leukoencephalopathy occur as reactivation of latent JC virus infection. PML manifests in severely immunocompromised patients, such as HIV-AIDS patients, especially those with her CD4 count less than 200, organ transplant recipients, patients with haematological malignancies, and more recently in developing patients.
Monoclonal antibodies such as natalizumab. In contrast to the expected reduction in PML incidence after initiation of antiretroviral therapy, an increase and exacerbation of pre-existing cases was noted as a result of immune reconstitution after antiretroviral therapy.
Pathophysiology
Gene sequence rearrangements in JC virus DNA are essential for reactivation of these latent forms, especially in immunocompromised states. Host responses play an important role in removing viruses. There is intense perivascular infiltration of immune cells, HIV antigens, and viral proteins, causing demyelination.
The cell-mediated immune response is an important component of the defense mechanism, especially cytotoxic T lymphocytes. Cytotoxic T lymphocytes were found to be elevated and dormant in her CSF samples of patients who shed virus. Cellular responses fail in conditions such as AIDS, making such patients susceptible to this demyelinating disease.
The focus of latent infection begins in oligodendrocytes in the CNS, and reactivation leads to a lytic infection that destroys the myelin they produce. This severe demyelinating leukoencephalopathy often manifests as CNS white matter lesions.
History and Physical
Evaluation for new neurological symptoms in patients with suspected or confirmed immunosuppression should include progressive multiple leukoencephalopathy as part of the differential diagnosis.
These patients may have altered mental status and abnormal neurologic examinations and should be evaluated. In AIDS patients, the level of immunosuppression, as evidenced by CD4 counts, is an index of the etiology that may underlie aberrant neurological symptoms.
If the CD4 count is less than 200, the PML will enter the differential. However, it can also occur with values above 200. The neuropathy that emerges correlates with areas of white matter demyelination. Symptoms of PML include progressive, multifocal, and subacute focal neuropathy, which vary according to the site of the lesion and include cognitive impairment, limb ataxia, gait ataxia, hemiplegia, hemianopsia, and aphasia. symptoms.
Areas commonly affected include the subcortical white matter, the periventricular region, and the cerebellar peduncle. In most cases, the optic nerve and spinal cord are not affected.
Evaluation
Routine CBC and HIV PCR tests are required to identify the cause of the immunocompromised state that led to reactivation of the JC virus. Evaluation of abnormal neurological findings in immunocompromised patients, such as those with AIDS, begins with radiographic imaging.
Contrast-enhanced imaging by CT or MRI helps clinicians determine the presence of inflammatory changes and masses. These findings are not seen in PML and their presence suggests alternative diagnoses such as Toxoplasma encephalitis or primary CNS lymphoma.
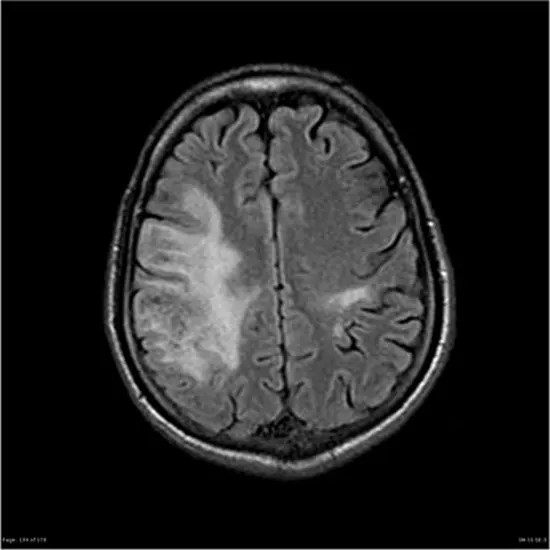
PML appears on CT as a low-density, confluent lesion without mass effect, contrast enhancement, or risk of herniation. On MRI, they appear as areas of decreased signal on T1-weighted images. Contrast-enhanced MRI shows hyperintense lesions on T2-weighted images, especially in subcortical lesions, periventricular regions, and lesions confined to the cerebellum.
On PML-IRIS, patients show worsening neurological status after initiation of ART or after dose reduction of immunosuppressive therapy, followed by contrast enhancement, edema, and inflammatory changes indicative of mass effect on MRI images. . Similar her MRI findings are seen in her patient who developed PML after natalizumab treatment.
CSF-JCV DNA isolation by PCR confirms the diagnosis of PML. In PML-IRIS, CSF DNA may be negative for JCV due to competent immune system defenses with the ability to block viral replication and are undetectable by viral assays.
Treatment / management
Currently, no effective treatment has been found to completely cure progressive multifocal leukoencephalopathy. Agents such as cidofovir, cytarabine, and mefloquine have been studied but have not shown clinical benefit in treating PML.
Treatment is currently guided by ongoing efforts to enhance the adaptive immune response, and ways to achieve this goal vary by clinical setting.
Immediate initiation of HAART is recommended for HIV patients. In transplant patients, the risk of transplant rejection may limit the use of multiple immunosuppressive therapies. For natalizumab-induced PML, treatment discontinuation and therapy to plasmapheresis were recommended.
Certain studies have investigated the benefits of using dendritic cell vaccines to boost adaptive immune responses. Dendritic cells labeled with JC antigen can generate significant CD8 responses and have been shown to prolong survival in PML patients. As these studies were performed in her three of her PML patients, further studies in larger groups are needed to justify the use of these agents.
Regarding the treatment of PML IRIS, an improvement in neurological status has been observed after cessation of ART therapy. Certain studies have shown good results when treated with steroids.
However, discontinuation of antiretroviral therapy can increase viral load and lead to antiretroviral resistance. PML IRIS carries a significant risk of masses and hernias, and if such complications occur, glucocorticoids can be used to counteract the damage caused by immune reconstitution.
Differential diagnosis
Considering the differences in the abnormal neurological examination of the immunocompromised patient, Toxoplasma encephalitis, primary CNS lymphoma, PML, HIV encephalopathy, CMV encephalitis are possible.
From a clinical perspective, PML can manifest as focal neurologic deficits of sensory, motor, or visual parameters. Therefore, this appearance resembles that of Toxoplasma gondii and primary CNS lymphoma, requiring radiological differentiation.
Although similar in appearance to PML, PML is radiologically asymmetric, well-demarcated, non-enhancing with no mass effect, whereas both Toxoplasma gondii and PCNL present as contrast-enhancing lesions. Also, his CSF testing at PCNL may show a positive result for Epstein-Barr virus.
Non-enhancing lesions are seen in PML, CMV encephalitis, and HIV encephalopathy. Therefore, these conditions should be distinguished from each other.
HIV encephalopathy often presents as cognitive deficits and no signs of focal neurologic deficits. In addition, radiological evaluation shows symmetric and poorly defined lesions. HIV encephalopathy also shows evidence of a positive HIV viral load on CSF testing.
CMV encephalitis is a differential disease to consider. The symptoms he resembles PML, with non-enhancing lesions seen on MRI. However, the radiological appearance differs from PML. MRI images show multiple diffuse micronodules in the cortex, basal ganglia, brainstem, and cerebellum.
Multiple sclerosis relapse in patients treated with natalizumab for MS requires differentiation from PML. Since both diseases are demyelinating processes, clinical signs and symptoms can overlap, making it difficult to distinguish between the two disease entities.
However, radioimaging is markedly different and may help identify natalizumab-induced PML. PML lesions are larger, hyperintense, and solitary compared to the multifocal, hypointense nature of typical MS lesions.
Prognosis
PML is a progressive, fatal disease. Currently, the main goal of treatment is to improve survival. Factors that enhance survival include low viral load of JC virus in PCR-CSF specimens, high CD4 count, and radiographic contrast enhancement.
In addition, initiation of antiretroviral therapy in AIDS patients is known to improve survival. A robust adaptive cell-mediated immune response is a good predictor of long-term survival, as evidenced by the presence of PML-specific CTL lymphocytes in the sera of patients who have recovered from PML.
Among transplant recipients who develop PML, those who undergo hematopoietic stem cell transplantation have been found to have lower mortality and higher survival rates than those who undergo solid organ transplantation.
For patients who developed natalizumab-induced PML, improved survival was observed in patients with certain prognostic factors, including younger age, lower JC viral load, less neurological dysfunction before treatment, and increased mass on contrast imaging.
Steroid use and contrast-enhanced MRI imaging were favorable prognostic indicators of survival in patients who developed PML-IRIS after antiretroviral therapy.
Complications
PML is a severe, progressive multifocal demyelinating disease that is fatal in most cases. Current treatments are aimed at prolonging survival. Remyelination does not occur and patients may develop long-term complications. These are primarily neurological and include cognitive, sensory, motor and coordination deficits.









