Unstable hemoglobins are a group of genetic disorders that affect the structure and function of hemoglobin, the protein responsible for carrying oxygen in the blood. Unlike normal hemoglobins, which have a stable structure,...
What are Unstable Hemoglobins?
Unstable hemoglobins are a group of genetic disorders that affect the structure and function of hemoglobin, the protein responsible for carrying oxygen in the blood. Unlike normal hemoglobins, which have a stable structure, unstable hemoglobins have mutations that cause them to become unstable and prone to breaking down, forming clumps, or precipitating out of the blood. While unstable hemoglobins are rare, they can have a significant impact on the health and quality of life of affected individuals and require careful management and monitoring.
What is Haemoglobins?
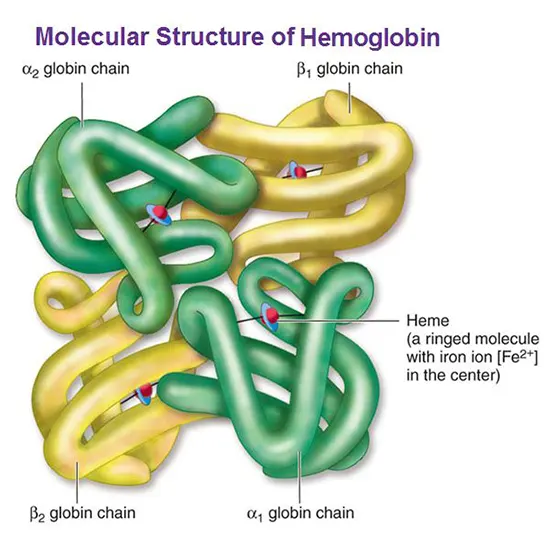
Hemoglobin is a protein present in red blood cells that plays a pivotal role in the carrying of oxygen throughout the body. It consists of four globin chains, each of which is bound to a heme group. The iron atom is responsible for binding to oxygen molecules, allowing hemoglobin to transport oxygen from the lungs to the body's tissues.
Hemoglobin is a complex protein with a unique structure that enables it to perform its essential function. Mutations in the genes that encode the globin chains can cause the production of abnormal hemoglobins, which may be unstable or behave differently than normal hemoglobin. These mutations can lead to a group of genetic disorders called hemoglobinopathies, which can affect the ability of red blood cells to transport oxygen efficiently.
Epidemiology of Unstable Haemoglobins
The exact prevalence of these disorders varies depending on the specific mutation involved and the population being studied.
One of the most well-known unstable hemoglobins is sickle cell hemoglobin (HbS), which is caused by a mutation in the beta-globin gene. Sickle cell hemoglobin is most commonly found in individuals of African descent, with an estimated 1 in 365 African Americans carrying the sickle cell trait, and 1 in 13 African Americans carrying the trait in some regions. Sickle cell disease, the most severe form of the disorder, affects around 70,000-100,000 Americans and is also prevalent in parts of the Middle East, India, and other regions with high rates of consanguinity.
Other unstable hemoglobins, such as HbE, HbC, and HbD, are found primarily in populations of Southeast Asian, Mediterranean, or African descent, and have lower overall prevalence rates. HbE, for example, is estimated to affect around 1 in 30 people in Thailand, while HbC and HbD are more common in populations of West African or Middle Eastern origin.
While unstable hemoglobins are relatively rare, they can have a significant impact, particularly in regions where they are more common. Early detection, genetic counseling, and appropriate medical management are essential for minimizing the risk of complications and improving outcomes for patients with these disorders.
Unstable hemoglobins are caused by mutations in the genes that produce the hemoglobin protein. These mutations can affect the structure or stability of the hemoglobin molecule, leading to a variety of functional abnormalities.
Etiology of Unstable Hemoglobins
Many noncovalent interactions inside the tetrameric hemoglobin molecule preserve the structural integrity of each member and link them to one another.
Hemoglobin can denature and precipitate as insoluble globins due to amino acid changes or indels that weaken noncovalent interactions. These globins can then attach to the cell membrane to form Heinz bodies.
Heinz bodies reduce the flexibility of erythrocytes, making it more difficult for them to navigate the splenic sinuses; they can also "pit," resulting in a loss of membrane area and ultimately the destruction of red blood cells, which manifests as hemolytic anemia.
Inheritance of Unstable Hemoglobins
These illnesses are autosomal dominant. The patient's red blood cells contain both hemoglobin A and unstable hemoglobin because they are heterozygotes. As the conditions are deemed fatal, homozygotes and compound heterozygotes are not seen.
Patients occasionally get a de novo mutation that results in unstable hemoglobin. More than 80% of patients have a globin chain deficiency. Errors in the α globin chain are less likely to induce a clinical disease since the genome has four α-globin genes, and a mutation in one gene leads to a low fraction of aberrant globin in the cell.
Causes of Unstable Hemoglobins
Sickle cell hemoglobin (HbS), which results from a single point mutation in the beta-globin gene, is the most often occurring unstable hemoglobin. Under specific circumstances, such as low oxygen levels or dehydration, this mutation causes the hemoglobin molecule to form long, stiff fibers, which results in the distinctive sickle-shaped red blood cells and several related issues.
Different mutations in the beta-globin gene or in other genes that make components of the hemoglobin molecule lead to other unstable hemoglobins, such as HbE, HbC, and HbD. A variety of symptoms and consequences can result from these mutations, which can also impair the hemoglobin molecule's stability or functionality.
In some instances, the inheritance of unstable hemoglobins may follow an autosomal dominant pattern, in which case the condition might exist with just one copy of the defective gene. In other instances, they might be passed down in an autosomal recessive pattern.
Rarely, instead of being inherited from a parent, unstable hemoglobins can also develop spontaneously as the result of a novel mutation in a person's DNA.
Generally, the precise reasons for unstable hemoglobins differ depending on the particular mutation involved, but they all lead to a disruption of the hemoglobin molecule's regular structure and function, which could have catastrophic consequences for those who are affected.
Unstable Haemoglobins can Lead to?
Hemoglobin is a complex protein made up of four globin chains, each of which is bound to a heme group. The two most common types of globin chains are alpha-globin and beta-globin, which combine to form different types of hemoglobin. In healthy individuals, hemoglobin is stable and functions normally to transport oxygen throughout the body.
However, mutations in the genes that encode the globin chains can cause the production of abnormal hemoglobins, which may be unstable or behave differently than normal hemoglobin. Hemoglobinopathies are a group of genetic disorders caused by these mutations, and they are usually inherited in an autosomal recessive pattern, meaning that a person must inherit two copies of the mutated gene to develop the condition.
Sickle cell anemia is caused by a mutation in the beta-globin gene that leads to the production of hemoglobin S (HbS), an abnormal form of hemoglobin. HbS is less soluble than normal hemoglobin, and it tends to form rigid, sickle-shaped structures when it gives up its oxygen molecule. These sickled red blood cells can clog small blood vessels and cause tissue damage, leading to a range of complications such as pain, organ damage, and an increased risk of infections.
Thalassemias are another group of hemoglobinopathies that result from a deficiency of one or more of the globin chains. For example, beta-thalassemia is caused by a mutation in the beta-globin gene that reduces or eliminates the production of beta-globin chains, leading to the formation of abnormal hemoglobin and a range of symptoms such as anemia, jaundice, and bone deformities.
In some cases, unstable hemoglobins can also cause hemolytic anemia, a condition in which erythrocytes (RBCs) are depleted faster than they can be replaced. This can occur when the unstable hemoglobin causes the red blood cell membrane to become more fragile, leading to premature rupture and destruction of the cell. Hemolytic anemia can cause symptoms such as fatigue, jaundice, and an enlarged spleen.
Clinical Features of Unstable Hemoglobins
- The degree of hemoglobin depletion can range from almost normal to significantly depleted.
- Patients may also have diminished to nonexistent haptoglobin, high indirect bilirubin, elevated lactic dehydrogenase, and reticulocytosis. The blood film exhibits basophilic stippling, polychromasia, anisocytosis, and poikilocytosis (Heinz bodies).
- Usually, hemolysis is made up for. Hemoglobin levels in the upper normal range are possible in patients who have unstable hemoglobins with high oxygen affinities.
- Hemolytic episodes may be triggered by oxidant medication therapy, highlighting the diagnosis.
- Because mutant chains (fetal hemoglobin) replace normal chains during the first year of life, chronic hemolytic anemia in-chain mutations become apparent after the neonatal period.
- Pallor, jaundice, and splenomegaly are possible physical findings.
- Some patients' urine is black, which is most likely the result of Heinz catabolism.
Laboratory Features of Unstable Haemoglobins
- The concentration of hemoglobin may be normal or low. The loss of hemoglobin via denaturation and pitting may result in a drop in the mean corpuscular hemoglobin.
- Hypochromia, poikilocytosis, polychromasia, anisocytosis, and basophilic stippling can all be seen on blood films.
- Circulating red cells frequently include Heinz bodies, following splenectomy, their frequency increases.
- Particularly in cases where the aberrant hemoglobin has a high oxygen affinity, reticulocytosis is frequently out of proportion to the severity of the anemia.
Diagnosis of Unstable Hemoglobins
- The hemolysate is incubated with an isopropanol solution at a concentration of 17% in an easy screening test known as the isopropanol precipitation test. When unstable hemoglobin variations are present in hemolysates, they cause a precipitate to form, as opposed to a clear hemolysate. This test for unstable hemoglobins has a high sensitivity but is not focused.
- The heat denaturation test is just as accurate as the isopropanol test but is more time-consuming and labor-intensive. Less people have access to it, though.
Erythrocytes must be stained with a supravital stain for the Heinz body detection procedure to be successful. Heinz bodies, however, are not just for hemoglobins with a volatile state.
- Hemoglobin electrophoresis: while potentially helpful, it is insensitive because a normal pattern does not completely rule out unstable hemoglobin. Electrophoresis is not a valid or accurate test for unstable hemoglobins, and as a result.
Due to differences in their hydrophobicity, certain unstable globin variants can be more specifically detected by Reverse phase high-performance liquid chromatography, though not always with 100% accuracy.
The right test to find unstable hemoglobins with changed oxygen-hemoglobin affinities is Hemoglobin oxygen affinity determination (P50O2).
Only DNA analysis allows for the precise identification of unstable hemoglobin mutation.
Differential Diagnosis of Unstable Hemoglobin
- All individuals with hereditary nonspherocytic hemolytic anemia should be evaluated for the possibility of unstable hemoglobin, particularly if they have hypochromic red blood cells and reticulocytosis that are out of proportion to their level of anemia.
- Not all patients who test positive for unstable hemoglobin have this condition; people with sickle hemoglobin, high methemoglobin, or hemoglobin F may also test false-positive for isopropanol stability.
- Unstable hemoglobins include hemoglobin H and hemoglobin Bart's. They are present in -thalassemia patients and can be discovered by electrophoresis.
Treatment of Unstable Hemoglobin
The treatment for unstable hemoglobins depends on the type of hemoglobinopathy and the severity of the condition.
Here are some common treatments for unstable hemoglobins:
Blood transfusions: For severe cases of a hemoglobinopathy, regular blood transfusions may be necessary to replace the unstable hemoglobin with normal hemoglobin. This can help alleviate symptoms such as anemia, fatigue, and jaundice.
Hydroxyurea: This medication can help increase the production of fetal hemoglobin, which is a type of hemoglobin that is more stable than adult hemoglobin. Hydroxyurea can reduce the frequency and severity of painful episodes in sickle cell anemia and may also reduce the need for blood transfusions.
Bone marrow transplant: For some types of hemoglobinopathies, a bone marrow transplant may be a potential cure. This involves replacing the patient's bone marrow with healthy bone marrow from a compatible donor. However, this procedure carries significant risks and is only suitable for a small number of patients.
Pain management: For individuals with sickle cell anemia, pain management is an essential part of treatment. Painful episodes, also known as crises, can be managed with pain relievers, hydration, and other supportive measures.
Oxygen therapy: In some cases, oxygen therapy may be helpful for individuals with hemoglobinopathies. This involves breathing in concentrated oxygen to increase the amount of oxygen in the blood, which can alleviate symptoms such as shortness of breath and fatigue.
It is essential to work closely with a healthcare provider to develop a treatment plan that is appropriate for each individual's needs.
Treatment for hemoglobinopathies may require a multidisciplinary approach involving specialists in hematology, pain management, and other areas of medicine. With proper treatment and management, individuals with hemoglobinopathies can live healthy and productive lives.
"Taking steps to manage unstable hemoglobins is an important part of maintaining your overall health. Always consult with your healthcare provider for personalized guidance and support."









