Because it is difficult to validate microbiological diagnoses, the global epidemiology of Haemophilus ducreyi infections is poorly understood. When syndromic care for genital ulcer disease (GUD) was implemented, the proportion...
Because it is difficult to validate microbiological diagnoses, the global epidemiology of Haemophilus ducreyi infections is poorly understood. When syndromic care for genital ulcer disease (GUD) was implemented, the proportion of genital and non-genital skin ulcers caused by H. ducreyi increased. Before 2000, 35 investigations conducted in 25 different countries found that the proportion of GUD brought on by H. ducreyi ranged from 0.0% to 69.0%. Following 2000, there were 14 studies conducted in 13 different nations with a range of 0.0% to 15.0%. Contrarily, H. ducreyi has recently been discovered to be a causal agent of skin ulcers in children in tropical settings; proportions ranged from 9.0% to 60.0% (6 investigations in 4 countries).
The fastidious gram-negative bacterium Haemophilus ducreyi is the cause of chancroid, a genital ulcer disease (GUD). The disease typically presents as several painful superficial ulcers linked to inguinal lymphadenitis, and the organism is typically disseminated during sexual activity via microabrasions.Asymptomatic carriage is uncommon since patients typically seek quick treatment due to the unpleasant nature of the lesions. In addition to producing GUD, H. ducreyi has been identified as a significant contributor to persistent skin ulceration in children from developing nations in several recent studies.
Chancroid's global epidemiology is poorly understood, and the World Health Organisation’s estimates of the incidence of treatable STIs around the world do not include it. Interpreting data on the epidemiology of H. ducreyi as a cause of GUD presents some significant issues. First off, a clinical examination can readily misdiagnose genital herpes patients as chancroid. Reports that are solely dependent on clinical diagnosis may thus be inaccurate. Furthermore, it is challenging to confirm clinical diagnoses due to the technical challenges of laboratory culture and the rarity of extremely sensitive and specific nucleic acid amplification techniques, such as PCR, outside of national reference laboratories or dedicated STI research settings.
Specimen Choice
Herpes simplex virus and syphilis tests should also be done in situations of suspected chancroid ).Chancroid causes vaginal ulcerative lesions, which are painful soft ulcers, and may cause bubo development, which is an inguinal lymph node swelling, however it is not known to spread throughout the body. As a result, material from ulcer lesions or bubo aspirates can be used as diagnostic tests to identify organisms or antigens. The 'gold standard' for diagnosing chancroid is still culture of the organism from ulcerative vaginal sores. However, it is only about 80% sensitive even when utilising the ideal mix of media. A swab collected from the bottom of the vaginal ulcer serves as the preferred specimen for the diagnosis of chancroid.
Method of Transport
H ducreyi can live for 2 to 4 hours on swabs despite the lack of an appropriate transport medium , therefore an alternative method—though less desirable—is to take the swab sample and put it in a transport medium like Amies. After that, the sample is transmitted to the lab as soon as feasible (8). Using a needle and syringe, pustular material from bubos should be aspirated through normal tissue (e.g., insert needle above bubo through normal tissue - this will minimise chronic fluid leakage and reduce contamination). Detection rates for cultures from intact bubos are significantly lower than those for cultures from the ulcer base or burst bubos.
The ulcer specimen should be injected onto two media that are ideal for H ducreyi growth at the bedside (see the section below on culture techniques for further information on this). The specimen from the ulcer swab can be transported dry in a sterile tube to be utilised for samples for amplification testing. The polymerase chain reaction (PCR) sample should be frozen at -70°C if the journey is expected to take a while. In the event that culture media is not accessible when the patient is seen, this is a useful backup . Ulcer material, freshly ruptured bubo exudate, and intact bubo exudate are the three most effective sources of H ducreyi for culture isolation.
Serum Diagnosis
There are currently no effective serological assays for the acute diagnosis of chancroid available in reference laboratories .For syphilis serology, blood that has been collected into a red stopper tube can be used.
Diagnosis
Microscopy
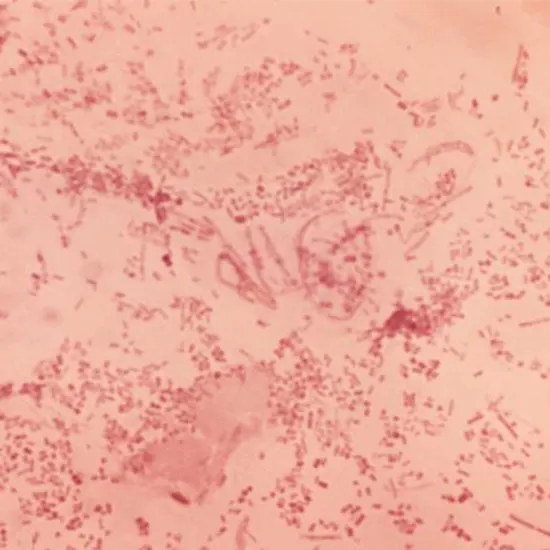
Microscopy has limited value due to its low sensitivity (5% to 63%) and specificity (51% to 99%) even if it is useful if there is a high load of organisms present that exhibit the distinctive Gram-negative coccobacilli in railway or chaining arrangement.
Antigen Detection
The use of a monoclonal antibody specific to the H ducreyi appears to be beneficial for direct immunofluorescent examination of ulcer material .An antigen detection technique for H ducreyi lipooligosaccharide (LOS) was created by Hansen et al. utilising a LOS-specific monoclonal antibody and a modified version of the limulus amoebocyte assay. These antigen detection techniques have sensitivity and specificity that range from 89% to 100% and 63% to 81%, respectively . These two antigen detection techniques do not, however, have commercially available reagents. Until a reference laboratory can be found to perform DFA, ulcer specimens for analysis may be collected, air dried, and preserved.
Culture
The only test method still used as the "gold standard" by the majority of laboratories is culture. Methods for amplification of nucleic acids are understood to be more sensitive. Multiple media should be used for best recovery .Having a split plate that contains the two types of media is the simplest way to deliver this mix of media. Gonococcal agar supplemented with 2% bovine haemoglobin and 5% foetal calf serum, 1% IsovitaleX supplement (BBL Microbiology Systems, USA) (Note: CVA [Gibco Laboratories, USA] was previously used, but is no longer available), and Mueller-Hinton agar supplemented with 5% chocolateized horse blood.
Nucleic Acid Detection
For culture confirmation or direct detection of H ducreyi in clinical samples, numerous genetic probe and amplification ( techniques have been developed. The Roche-created multiplex-PCR test (Roche Diagnostics Canada), which simultaneously identifies H ducreyi, T pallidum, and herpes simplex virus (17), is particularly pertinent. According to the PCR amplification data that have been made public so far, this method is more sensitive than the other culture methods that are currently available (1,7,8,17). But none of these approaches are presently offered on the marketplace. The specimen should be sent to the National Microbiology Laboratory in Canada so that a multiplex-PCR test can be performed for H ducreyi, H simplex, and T pallidum.
Serology
It has been demonstrated that patients with H ducreyi infections exhibit hemoral immune responses. Numerous cell components, including as whole cell lysate, pure and recombinant outer membrane proteins, and LOS, have been linked to the detection of antibody synthesis . For all of the antigens examined, the reaction frequently takes time to manifest, is cross-reactive with different Haemophilus species, and may persist for a considerable amount of time after the infection has subsided. The currently published assays have limited relevance as diagnostic tests for chancroid, especially in locations where chancroid is endemic, and are most valuable as epidemiological tools.
Summary
It is beneficial to put the diagnostic lab to the test by sending vaginal ulcer samples provided as swabs in transport media that have been made using stock cultures of H ducreyi. This will reveal any issues with the availability of chancroid culture media or culture confirmation. In contrast to new clinical isolates, stock isolates that have undergone extensive in vitro passages are frequently simpler to grow and identify.
These proficiency challenges may therefore not be very useful. To guarantee that selective antibiotics are working as intended, routine quality assurance should be carried out on all media provided to diagnostic clinics for the culture of suspect chancroid genital ulcer specimens. If requested media is created, the specimen inoculation and quality check should be done simultaneously.









