The term "carcinoid syndrome" refers to a collection of symptoms brought on by the systemic release of various humoral factors, primarily from well-differentiated neuroendocrine tumours, including polypeptides,...
What is Carcinoid Syndrome?
The term "carcinoid syndrome" refers to a collection of symptoms brought on by the systemic release of various humoral factors, primarily from well-differentiated neuroendocrine tumours, including polypeptides, biogenic amines, and prostaglandins. Carcinoid tumours were previously used to describe well-differentiated neuroendocrine tumours.
Enterochromaffin cells, which are present in every part of our body, give rise to neuroendocrine tumours. Only about 10% of neuroendocrine tumours, according to reports, develop into carcinoid syndrome.
Etiology
The most frequent cause of carcinoid syndrome is liver-metastasising neuroendocrine tumours of the midgut. Rarely, foregut and hind-gut neuroendocrine tumours can also lead to carcinoid syndrome. About 70% of neuroendocrine tumours develop in the GI system, with the respiratory tract accounting for the remaining 25%. Rarely, neuroendocrine tumours can develop in other organs like the kidneys, sperm, or ovaries.
Epidemiology
-
The prevalence of neuroendocrine tumours is comparatively low. Carcinoid syndrome develops in only 10% of neuroendocrine tumours, as was previously stated. The age-adjusted prevalence of non-pancreatic neuroendocrine tumours is 4.7 per 100,000, according to Surveillance, Epidemiology, and End Results (SEER).
-
Neuroendocrine tumours are becoming more commonplace. Between 1973 and 2004, the prevalence of neuroendocrine tumours—including non-pancreatic and pancreas neuroendocrine tumours—rose from 1.09 to 5.25/100,000. (Per data from SEER). The rise in endoscopic and radio-imaging investigations over the last few decades is probably to blame for this rise in incidence. Racial and female differences in incidence.
-
. According to recent statistics, black males are more likely than Caucasians to develop neuroendocrine tumours. Males and females have nearly equal rates of tumour incidence, with a small male advantage. The average age at which neuroendocrine tumours are diagnosed is between 55 and 60.
Pathophysiology
Biologically active amines and peptides that reach the systemic circulation and avoid the liver's first-pass metabolism are the basis for the pathophysiology of the carcinoid syndrome. These functional substances are typically inactivated in the liver. However, when neuroendocrine tumours with liver metastases are involved, either these bioactive substances reach the systemic circulation directly or they are not rendered inactive by abnormal liver function.
Less commonly, conditions like primary gut tumour with extensive retroperitoneal nodal metastases, ovarian tumour, or bronchial carcinoid, which release bioactive amines straight into the systemic circulation, can cause carcinoid syndrome without liver metastasis.
About 40 different kinds of biologically active amines and peptides are released by neuroendocrine tumours. Serotonin, histamine, tachykinins, kallikrein, and prostaglandins are the most prevalent ones. Most of the clinical characteristics are caused by serotonin, which is a by-product of the breakdown of tryptophan.
Only 1% of the nutritional tryptophan is typically converted into serotonin. However, up to 70% of tryptophan is converted into serotonin in neuroendocrine tumours. Aldehyde dehydrogenase aids in the oxidative processes that cause serotonin to produce 5-hydroxy indoleacetic acid (5-HIAA), which is then excreted in the urine.
Serotonin increases the gastric tract's motility and secretion, which results in diarrhoea. Neuroendocrine tumours cause a deficiency of tryptophan, which is required for the synthesis of niacin, as most of the tryptophan is diverted to the serotonin formation route. Niacin insufficiency consequently causes Pellagra, which presents as a trifecta of diarrhoea, dementia, and dermatitis. Additionally, prostaglandins contribute to the gastrointestinal tract's higher fluid secretion and intestinal motility, which results in diarrhoea.
The enzyme aromatic L-amino acid decarboxylase, which metabolises 5-hydroxytryptophan to serotonin, is absent from neuroendocrine tumours of the foregut and lungs. As a result, serotonin is not produced by neuroendocrine tumours of the foregut and lungs. On the other hand, neuroendocrine tumours in the hindgut typically don't make any functional hormones.
Symptoms and physical evaluation
Carcinoid syndrome frequently manifests as vasodilatory effects of biologically active amines, peptides, and prostaglandins (flushing, wheezing), gastrointestinal symptoms (diarrhoea, malabsorption), Pellagra (secondarily caused by niacin deficiency), cardiac symptoms (right-sided valvular disease, primarily tricuspid regurgitation), fatigue, and occasionally cognitive impairment.
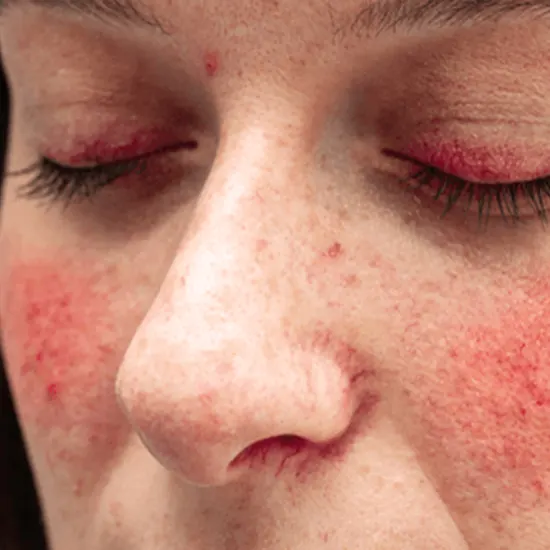
In about 85% of patients with carcinoid syndrome, flushing is the most prevalent presenting symptom. It is characterised as a paroxysmal discolouration of the upper body's skin, including the face, neck, and upper trunk, that can last for up to 30 minutes and ranges in colour from salmon pink to dark red. It can be brought on by eating, drinking alcohol, tension, palpating the liver, and anaesthesia, in addition to occurring spontaneously. Midgut neuroendocrine tumours are mainly linked to it.
About 80% of the time, there is diarrhoea. Diarrhoea is typically described by patients as abrupt, non-bloody, and watery. Up to 30 times a day are possible. In cases of carcinoid syndrome, diarrhoea is typically persistent. Cramping in the stomach is possible. Flush-related diarrhoea is not always the case.
Up to 60% to 70% of individuals have cardiac involvement. The endometrium, valves, chambers, pulmonary, and aortic arteries may develop masses of fibrous tissue that resemble plaques. The signs and symptoms of valvular heart disease or heart failure may be evident in patients.
Other, less frequent symptoms include Pellagra, muscular atrophy, and ureteral obstruction brought on by retroperitoneal fibrosis.
Gastric and pulmonary neuroendocrine tumours can result in some unusual symptoms. Due to the release of histamine, gastric neuroendocrine tumours can result in cherry red, serpiginous, spotty flushing that is well-defined and accompanied by severe itching. In addition to anxiousness, altered mental state, and tremors, lung neuroendocrine tumours can produce severe and protracted flushing that lasts for hours or days. Although the precise reason is unknown, it might be connected to histamine release.
Evaluation of carcinoid syndrome
Initial biochemical indicators are tested for in the diagnostic process for carcinoid syndrome, and then radiographic and endoscopic studies are used to localise tumours.
Testing for biochemical markers
- The original diagnostic test is a 5-HIAA urine analysis after twenty-four hours. The final by-product of serotonin breakdown is 5-HIAA. Both the sensitivity and specificity of this pee test are 90%. Tryptophan-rich foods like bananas, pineapple, plums, kiwis, avocados, eggplant, pecans, and walnuts can cause a false result. The 5-HIAA level can be raised by number of drugs, including acetaminophen, guaifenesin, coffee, nicotine, methamphetamine, and phenobarbital. Aspirin, ethanol, heparin, imipramine, levodopa, methyldopa, monoamine oxidase (MAOI), INH, and corticotropin, on the other hand, can lower the amount of 5-HIAA. For at least 24 hours prior to and throughout urine collection, patients should abstain from these meals and medications. In order to monitor therapy effectiveness, the 5-HIAA can be used to correlate tumour burden.
- A glycoprotein called chromogranin A is produced by neuroendocrine tumours. It is helpful for identifying non-secreting tumours. Its sensitivity is excellent, but its precision is subpar. It serves as a fantastic cue for further action. A significant elevation of CgA may also result from the use of proton pump inhibitors, atrophic gastritis, renal failure, hyperthyroidism, heart failure, HTN, or prostate cancer.
- Blood serotonin testing is typically not advised due to the unreliable precision of this procedure.
- Although convenient, the plasma 5-HIAA quantity has not yet been proven.
- Studying Localisation and Staging, if biomarkers are present, different radiographic imaging techniques and/or endoscopic methods can be used to determine the location of the tumour.
- Cross-sectional imaging with a triple-phase computer tomography image, magnetic resonance imaging, and somatostatin receptor scintigraphy are all examples of radiographic imaging. The preferred diagnosis procedure is a CT scan of the abdomen (with triphasic CT of the liver). Because MRI is more sensitive to liver metastases than CT, some people favour MRI.
- Although not accessible in the United States, 24-hour urine testing for serotonin may be helpful in foregut neuroendocrine tumours with a rare carcinoid syndrome brought on by 5-HT.
- The specificity of indium-111 pentetreotide (Octreoscan) is greater than 90% in symptomatic patients and between 80% and 90% in asymptomatic patients. Its specificity is low, and it is unable to identify poorly differentiated neuroendocrine tumours. It is recommended to use functional PET imaging with 68-Ga Dotatate because it is a more recent modality with higher sensitivity and resolution for a small tumour.
- For bronchial neuroendocrine tumours, bronchoscopy with sampling is an option. Upper or lower endoscopy or an ultrasound-guided biopsy for histopathology may be carried out if radiographic results are positive, depending on the location of the illness. The tissue collected could be examined for neurone-specific markers like enolase, Chromogranin A, the mitotic index Ki-67, saprophytic, and serotonin.
Treatment/Management
Somatostatin analogues, liver-directed therapy, surgical debulking for early-stage, low-grade neuroendocrine tumours, chemotherapy for poorly differentiated neuroendocrine tumours, and refractory carcinoid syndrome are some of the different treatment modalities available for carcinoid syndrome.
Whether there are metastases or not, surgery is essential in the management of carcinoid syndrome. Always think about surgically removing the main tumour as well as any nodal and liver metastases to lessen the tumour burden.
Medical management
Somatostatin is an amino acid peptide that is an inhibitory hormone that is synthesised by paracrine cells found ubiquitously throughout the gastrointestinal tract. It inhibits the release of most gastrointestinal, endocrine hormones. About 80% of neuroendocrine tumours have somatostatin receptors. There are two somatostatin analogues available for medical management: Octreotide and Lanreotide.
The long-acting medication lanreotide is given at a dose of 60 mg to 120 milligrammes every four weeks. It works similarly to octreotide.
In between 50% and 70% of patients, both somatostatin analogues offer symptomatic relief, and between 40% and 60% of patients experience biochemical reactions. Numerous studies have revealed that Octreotide and Lanreotide also prevent the growth of tumour cells.
Steatorrhea, which is brought on by pancreatic malabsorption, nausea, stomach bloating, and abdominal pain are the most frequent adverse effects connected with somatostatin analogues. Pancreatic enzyme supplements frequently aid in the relief of uncomfortable feelings. Patients are at risk for developing biliary sludge and gallstones due to the gallbladder's decreased motility and contraction brought on by the inhibitory effect of somatostatin, which must be addressed with patients prior to beginning therapy.
Surgical management
Surgery to remove the tumour completely cures carcinoid syndrome in patients with bronchial neuroendocrine tumours who had it at an early period of development. Patients who have hepatic metastases that can be surgically removed, surgical resection, or partial hepatectomy experience a reduction in symptoms. Palliative cytoreductive or tumour debulking surgery has been shown to ameliorate symptoms, morbidity, and mortality in patients with severe tumour burden and widely metastatic disease. To avoid the potential side effects of somatostatin analogue therapy, such as biliary slugging and gallstones, an elective cholecystectomy can also be given during surgery. Early gastric and rectal neuroendocrine tumours that are endoscopically removed and are less than 1 cm in size may result in full recovery from carcinoid syndrome.
Replacement of the tricuspid valve may increase mortality in individuals with severe tricuspid regurgitation and cardiac neuroendocrine tumours.
Percutaneous hepatic transarterial embolization is an option for patients who cannot have surgery but have a significant number of tumours, particularly liver metastases. For embolization, radiolabeled yttrium 90 (Y) resin or glass microspheres have also been used, but their long-term danger has not been determined.
Differential diagnosis:
The broad range of differential diagnoses for carcinoid syndrome can be attributed to the syndrome's tendency to present with a variety of clinical features. The key differential diagnoses listed below should be taken into account when making a diagnosis of carcinoid syndrome:
- Rheumatoid bowel disease
- abnormalities of gastrointestinal motility
- Celiac illness
- Anaphylaxis
- Extreme hives
- Angioedema
- Ogilvie disease
Summary
Although carcinoid syndrome is an uncommon condition, it has a high morbidity and mortality rate. An inter-professional team made up of an oncologist, endocrinologist, internist, surgeon, radiologist, and pathologist is best suited to managing the condition. Somatostatin analogues, liver-directed therapy, surgical debulking for early-stage, low-grade neuroendocrine tumors, chemotherapy for poorly differentiated neuroendocrine tumors, and refractory carcinoid syndrome are some of the different treatment modalities available for carcinoid syndrome.
Surgery is essential in the management of carcinoid disease, whether or not there are metastases. To lessen the tumour load, always think about performing surgical resection of the main tumour as well as any nodal and liver metastases.
Once tricuspid valve insufficiency has been ruled out by a diagnosis of carcinoid syndrome, the nurse practitioner or primary care physician should make sure the patient has an echocardiography. For final management, these patients must be transferred to a cardiologist. Patients with carcinoid syndrome have varying prognoses depending on the underlying reason; malignant cases have poor prognosis.









